Life in primary care in 2023 is tough: there are workforce shortages (Deakin, 2022), an increased number of consultations (British Medical Association, 2022) and, perhaps the most challenging, a massive backlog of routine care (Oliver, 2022).
One thing the pandemic has reminded us of is that healthcare needs are far from uniform. Some people or communities are at greater risk of harm than others (Williamson et al, 2020). With limited resources, in a publicly-funded healthcare service, we need to focus our efforts on patients who are at the highest risk of harm. Cost-effective tests need to be used that can identify people at risk of poor and expensive outcomes.
The ‘canary in the coal mine’ is a term that comes from the old mining practice of using caged canary birds, who are extremely sensitive to carbon monoxide, to warn the miners of the impending danger of rising levels of that gas. Chronic kidney disease (CKD) can be seen as a similar early warning sign that all is not well in the cardiovascular system of the person being treated. CKD is a common condition, affecting 5% of people aged 75 and over, similar in prevalence to other conditions, such as diabetes (NHS Digital, 2016). It is defined by abnormalities of kidney structure or function, persistent for more than 3 months, with implications to health (KDIGO, 2013). The financial cost, based on data already acquired over 10 years ago, is £1.45 billion (Kerr et al, 2012). Most of these costs are for renal replacement therapy, such as dialysis. The rate of dialysis continues to increase year on year (UK Renal Registry, 2021) and so this condition is financially expensive. The quality of life for a person living with CKD is massively reduced (Nguyen et al, 2018) and mortality is also increased. The most common cause of CKD in the UK is diabetes. The combination of developing CKD with diabetes is associated with up to a 16 year decrease in life expectancy (Wen et al, 2017). There is a close association between advancing CKD and increased hospital admission, particularly from cardiovascular causes, such as heart failure (Kalantar-Zadeh et al, 2021). Cardiovascular disease remains the leading cause of mortality in people with CKD (National Institute of Health and Care Excellence (NICE), 2021a), with approximately 10% of people with CKD surviving to renal replacement therapy (United States Renal Data System (USRDS), 2020).
‘Times are a changing’ in the management of CKD
The guardian of cost-effective care in the UK (except Scotland) is NICE, which has recently been very active in this area, producing new and updated CKD (NICE, 2021a) and type 2 diabetes guidelines (NICE, 2022e). In addition, we have seen a technology appraisal from NICE that looks at the role of dapagliflozin in both people with and without diabetes, advocating the role of this agent in a broad group of individuals (NICE, 2022b). For many years, guidelines have changed very little; primarily promoting risk factor management (blood pressure, lipid management and smoking cessation) in people living with diabetes (blood glucose) as well as the utilisation of kidney protective agents, such as angiotensin receptor blockers (ARB)/angiotensin-converting enzyme inhibitors (ACE-I), to prevent a decline in kidney function, progression to end-stage kidney disease and reduce cardiovascular morbidity and mortality.
The major change in these guidelines, compared to previous iterations, is the prominent role of sodium-glucose co-transporter 2 inhibitors (SGLT2i) in the management of CKD. This perhaps will surprise those not watching this space closely. The change comes after the publication of two large randomised controlled trials using canagliflozin (Perkovic et al, 2019) and dapagliflozin (Heerspink et al, 2020). These were the first large-scale kidney outcome trials for more than 20 years to show positive results.
NICE now recommends that after treatment with the maximum tolerated doses of ARB/ACE-I, an SGLT2i should be considered in patients who have an albumin to creatinine ratio (ACR) of 30 mg/mmol, and meet the criteria of the marketing authorisation (including eGFR), as a co-morbidity of type 2 diabetes, and specifically for those without diabetes, but living with CKD, dapagliflozin should be offered to those with an ACR ≥22.6 mg/mol.
The dapagliflozin in patients with chronic kidney disease trial (Heerspink et al, 2020), often abbreviated to the DAPA-CKD trial, is particularly noteworthy. This trial, using 10 mg of dapagliflozin, recruited people with albuminuric CKD, and an ACR of ≥22.6 mg/mol. Of the 4304 participants, approximately two-thirds had type 2 diabetes, with the remaining one-third not having diabetes of any type. The overall results showed a 39% reduction in the composite primary endpoint (50% reduction in estimated glomerular filtration rate (eGFR), end-stage renal disease or death from kidney or cardiovascular causes), with a numbers needed to treat (NNT) of 19 over the 2.4 year timeline of the trial. In addition, there was an absolute reduction in death from all causes with a NNT of 48. There was no statistical difference in the magnitude of benefits to participants regardless of their diabetes status. In short, dapagliflozin reduced renal decline and death in people living with CKD, regardless of whether they had type 2 diabetes or not.
Along with this evidence, there is a another drug, finerenone, a non-steroidal mineralocorticoid antagonist (ns-MRA), which has been licenced in the UK and is currently being appraised by NICE (2022b), with evidence that it reduces the decline in kidney function. This is shown in two large randomised clinical trials (Bakris et al, 2020). As things continue to develop, the outlook for people living with CKD is improving. The challenge is not only keeping up with the changing evidence base but also currently implementing those recommendations in the real world.
Patient identification in primary care
CKD is typically asymptomatic and so, in the early stages, effective screening is key to identifying people early in the disease. There is a ready availability of NICE-recommended treatments, not only to help preserve kidney function but also to reduce the cardiovascular burden in these vulnerable individuals. At present, the need is great, as nearly half of the people living with CKD remain undetected (Hirst at al, 2020).
NICE recommends two tests to screen people at high risk of CKD, these tests are:
Both tests are needed to fully assess not only whether the person has CKD but also to predict the risk of progression of CKD and the associated risk of cardiovascular events.
Currently, the testing of urine for CKD is sub-optimal (National Kidney Disease Audit, 2017). It is worth pointing out that if we are looking to diagnose just on an eGFR below 60 then patients will have already permanently lost half of their kidney function. This underlines the importance of urine testing, and is particularly true in people living with diabetes, where raised urine albumin is typically found years before the decline in the eGFR test (Tonneijck et al, 2017).
When to refer a patient to a nephrologist
Primary care clinicians need to work within their areas of competence and refer appropriately when help is needed, or when the patient requires specialist assessment and treatment. CKD is no different, and as previously mentioned, NICE (2021a) has recently updated its guidance on CKD. As well as changes in treatments, there has been a shift in who to refer to nephrology. The biggest change is to move away from a simple eGFR cut-off of 30 ml/min/1.73m², towards a risk-based approach, with the UK being the first country to recommend the use of the four-item kidney failure risk equation (KFRE). To calculate the risk using the KFRE four items are needed:
NICE also gives recommendations on how to communicate information to patients, reminding on the use of jargon-free language and giving enough time during the appointment to communicate and deal with questions. If the KFRE is greater than 5% over 5 years, a referral to a nephrologist is now recommended to be considered.
Additionally, NICE (2021a) recommended criteria for referral to a nephrologist that include:
When considering referral to a nephrologist, NICE (2021a) also recommends a shared decision making approach.
Effective management of potassium
One of the challenges of implementing guidelines to manage CKD is the risk of hyperkalaemia (high serum potassium), as those living with CKD, heart failure and diabetes are particularly at risk of hyperkalaemia (Sidhu et al, 2020). While drugs acting on the renin-angiotensin-aldosterone system (RAAS), such as ARB, ACE-I and MRAs have an important role in the prevention of the progression of CKD, the effective treatment of hypertension and the prevention of heart failure all can increase the risk of hyperkalaemia.
Hyperkalaemia frequently limits the use or titration of RAASi drugs (Epstein, 2011). ARB/ACE-I drugs, particularly when combined with MRAs, significantly increase the risk of hyperkalaemia. This is an important issue, not only due to the risks of high potassium but also because suboptimal dosing of RAASi drugs is associated with increased mortality (Epstein, 2015) and morbidity (Ouwerkerk, 2017). Therefore, to effectively manage CKD, diabetes and heart failure in primary care requires careful monitoring of potassium and management of hyperkalaemia.
Frequency of testing for hyperkalaemia
To identify patients with hyperkalaemia, they first need to be tested. The frequency of testing is positively associated with the detection of hyperkalaemia (Chang, 2016). Unfortunately, monitoring of potassium is suboptimal in UK clinical practice, leading to avoidable harms (Furuland, 2018). The NICE (2021a) guidelines for the management of CKD give a clear recommendation on urea and electrolyte (U+E) monitoring (Table 1). It is important to highlight two aspects of the recommendations; first, the frequency is dependent on KDIGO CKD stage (2013), which, in turn, further highlights the importance of urine ACR testing as part of CKD management, and, second, this is the suggested minimum frequency of testing, with increased testing indicated in acute illness.
| ACR category A1: normal to mildly increased (less than 3 mg/mmol) | ACR category A2: moderately increased (3 to 30 mg/mmol) | ACR category 3: severely increased (over 30 mg/mmol) | |
|---|---|---|---|
| GFR category G1: normal and high (90 ml/min/1.73 m2 or over) | 0 to 1 | 1 | 1 or more |
| GFR categorgy G2: mild reduction related to normal range for a young adult (60 to 89 ml/min/1.73 m2) | 0 to 1 | 1 | 1 or more |
| GFR category G3a: mild to moderate reduction (45 to 59 ml/min/1.73m2) | 1 | 1 | 2 |
| GFR category G3b: moderate to severe reduction (30 to 44 ml/min/1.73 m2) | 1 to 2 | 2 | 2 or more |
| GFR category G4: severe reduction (15 to 29 ml/min/1.73 m2) | 2 | 2 | 3 |
| GFR category G5: kidney failure (under 15 ml/min/1.73 m2) | 4 | 4 or more | 4 or more |
Management of patients receiving RAASi drugs
The UK Kidney Association (for hyperkalaemia) gives clear and evidence-based guidance regarding monitoring potassium levels and renal function when considering initiating, and titrating, RAASi drugs (this is summarised in Box 1).
The UK Kidney Association guidelines for the management of CKD advises U+E testing before initiation and within a week of starting, as well as at every titration. The frequency of monitoring of stable patients who are taking steroidal MRAs (eg spironolactone and eplerenone) should also be highlighted, specifically, to check U+E before initiating any RAASi drug, including mineralocorticoid antagonists. If the potassium is above 5 mmol/l, ACEI-I or ARBs should only be initiated with caution. Steroidal MRAs should not be initiated if serum potassium is above 5 mmol/l, and/or if the eGFR is less than 30 ml/min/1.73m². After initiation/titration all RAASi drugs U+E should be checked 1 to 2 weeks later and then monthly for the first 3 months, 3-monthly for the first year and 4-monthly thereafter.
These agents are increasingly being used in people living with CKD to manage co-morbid heart failure and hypertension. It is the author's experience that the frequency of testing of eGFR, particularly with stable patients, falls below those recommended by the UK Kidney Association (Alfonzo et at, 2020). SGLT2is (eg dapagliflozin) are indicated for the management of CKD and recommended by the guideline (NICE 2022e) for the management of CKD associated with type 2 diabetes. It is also recommended for some people with CKD outside the context of diabetes (NICE, 2022a), as well as for the management of type 2 diabetes and heart failure (NICE, 2021b). These agents (canagliflozin, dapagliflozin, empagliflozin and ertugliflozin) are associated with a reduction of hyperkalaemia events seen in clinical trial events vs placebo (Neuen et al, 2022). Co-prescribing of SGLT2i with RAASi drugs, when indicated, may not only improve cardiac and renal outcomes but also improve the ability to maintain maximal RAASi doses by limiting the associated risks of hyperkalaemia.
Clinical management of hyperkalaemia in the community
The UK Kidney Association (Alfonzo et al, 2020) gives clear, evidence-based recommendations on how to manage hyperkalaemic events (Figure 1). After the detection of potassium levels above 5.5 mmol/l, it is important to first consider whether this could be related to delayed transit time to the laboratory or indeed related to the phlebotomy technique. This should be repeated within 3 days if this is likely.
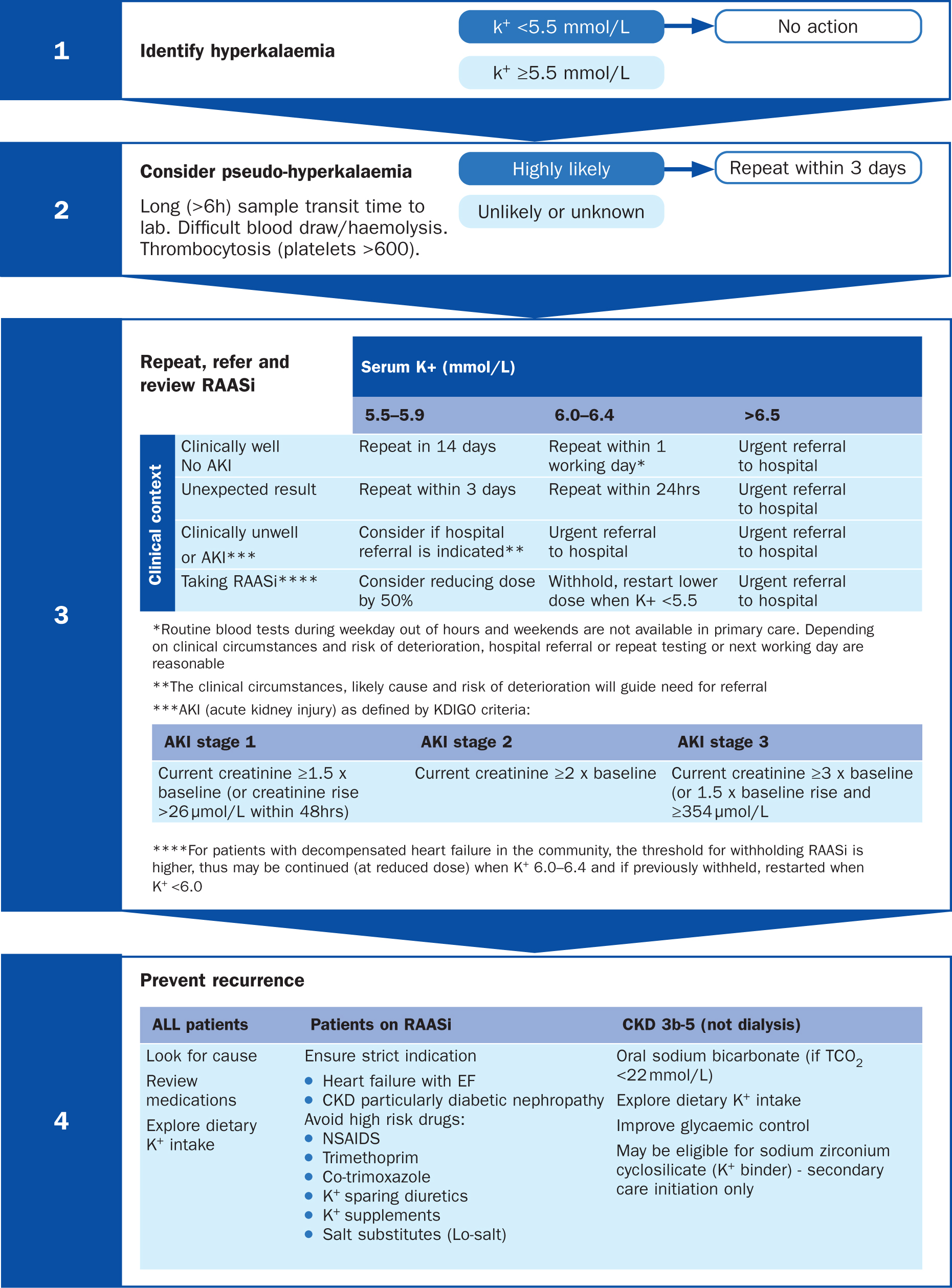
The clinical review of the patient is important for people with potassium between 5.5 and 6.5 mmol/l as this will determine the need for admission and repeat testing. In addition, a review of the current dosage of RAASi medications should be performed. A serum potassium above 6.5 mmol/l typically will need prompt hospital assessment and management.
To help prevent a recurrence of the high potassium it is important to review concomitant medications that could increase potassium levels, such as non-steroidal anti-inflammatory drugs (NSAIDS) and trimethoprim. Dietary potassium supplements, particularly the use of salt substitutes, should also be assessed and discussed with the patient. Consideration of whether the addition of an SGLT2i is indicated to manage CKD or other co-morbid conditions should also be considered at this point.
The use of potassium binders (eg patiromer or sodium zirconium cyclosilicate) reduces serum potassium and may facilitate continued evidence-based use of RAASi drugs. These are recommended by NICE technology appraisals for patiromer (NICE, 2022c) and sodium zirconium cyclosilicate (NICE, 2022d) in a specific group of patients, namely people with persistent hyperkalaemia and stages 3b to 5 CKD or heart failure, if they:
The UK Kidney Association recommends these are initiated by the specialist team (Alfonzo et al, 2020). This could be done through remote consultation or ‘advice and guidance’ depending on local pathways.
Conclusion
The financial and clinical burden of CKD is substantial and rapidly changing. To optimise care, it is important to work at scale to identify people living with the condition early and manage the risk factors for the progression of CKD. Alongside this, the associated cardiovascular disease needs to be monitored in order to serve our communities well. In addition to this, renal protective agents like RAASi, SGLT2i and non-steroidal MRAs should be introduced depending on need, while avoiding and/or treating hyperkalaemia. With the scale and complexity of CKD, timely access to specialist care is increasingly required. Referral criteria have changed, with more focus on the 5-year risk of developing end-stage renal disease by using the four-variable year KFRE.


